Electroscopes of Cheneveau and Laborde (ca. 1910-1920)
Based on the ideas of Pierre Curie, this electroscope was developed by C. Cheneveau and A. Laborde sometime around 1908 ("Appareils pour la Mesure de la Radioactivite, d'apres la Methode Electroscopique." Bulletin des Seances de la Société Francais de Physique, pp. 262-275, 1908). It undoubtedly served as the inspiration for the Lind electroscope. Samuel Lind worked with Marie Curie and was almost certainly familiar with this instrument.

In the early days, electroscopes were the most commonly used instruments to measure and detect radioactivity and this is one of the first electroscopes specifically designed for that purpose. Even better, the design is attributed to none other than Pierre Curie. And look at its condition—spectacular!
The bottom of the electroscope proper is connected to an interchangeable ion chamber the design of which depends on whether the sample being analyzed was a solid or gas (e.g., radon).
The chamber in the above photo was intended for a solid sample that would be placed on the tray attached to the door. Of course, the latter would be closed for the duration of the measurement.

As seen in the image to the right, a "microscope" is suspended from the top of the electroscope. This permits a more precise measurement of the deflection of the aluminum leaf (missing in our example). The wire screens pressed against the glass sides are there to shield the leaf from the build-up of a static charge.
The instrument in the top photo was made by L Deffez at 26 Rue Boulard, Paris XIV (see photos below left).
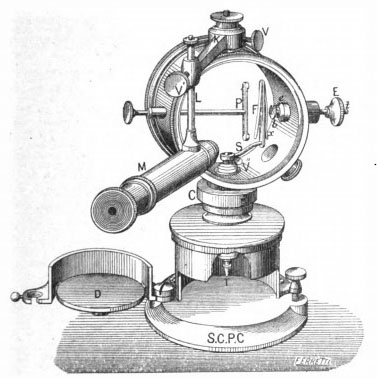
The other Pierre Curie electroscope in the ORAU collection, seen in the photo to the right, was made by the Societe Centrale de Produits Chimiques, 44 rue des Ecoles, Paris.
In the photo above right, the charging rod comes in from the left side at the 9 o'clock position. When the rod is pushed, its tip presses against the spring plate on the back (left) side of the vertical support rod to which the aluminum leaf would be attached. Coming in from the right side of the electroscope is a vertical cover plate (seen at the tip of the microscope) that would protect the leaf when the electroscope was being transported. A flask containing a desiccant (e.g., phosphoric anhydride) was once connected at the 7 o'clock position but unfortunately it broke off—you can see the glass fragments of the flask's neck.
The figure below right is taken from the original paper by Cheneveau and Laborde that describes this instrument.


I would like to express my thanks to Jean-François Loude for his assistance.
