Institutional Review Board Timeline
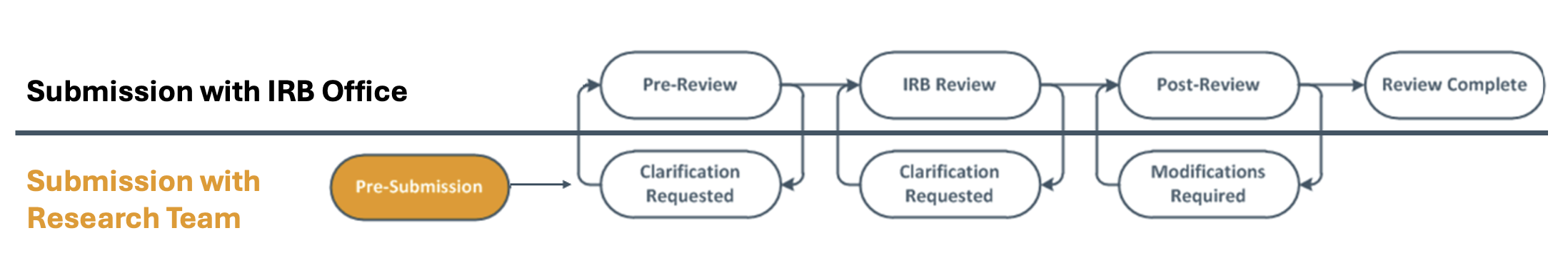
The graphic below illustrates when a study is with the Researcher and IRB office. The graphic of the workflow is found on your study’s workspace in the IRB electronic system.
Factors Affecting IRB Review
There are several elements that affect the overall IRB timeline. Those elements include, but are not limited to:
- Submission quality (e.g., incomplete sections in application, missing documentation, vague responses, etc.)
- Responsiveness of the study team to requests for clarification or changes
- Time required for IRB members to complete a comprehensive review of all submitted materials
- Volume of submissions to the IRB
- Studies are reviewed in the order they were received
- Need for a Reliance Agreement (e.g. IAA) or Data Use Agreement
- Completion of training for principal investigator and study staff (i.e., members of the research team who are involved in the design, conduct, or reporting of the research; or who participate in those aspects of the research that involve contact with human subjects or their data or biospecimens).
IRB Turnaround Times
These estimates include the time required for pre-review, investigator/study team responses, and designated or fullboard review.
These estimates are based on studies reviewed last calendar year.
Initial Study:
- Exempt: 29 days
- Expedited: 14 days
- Full Board: None
Modification:
- Exempt: 10 days
- Expedited: 11 days
- Full Board: None
Note: Due to the time required to review all studies submitted to the IRB, repeated calls or emails to the IRB may delay the review process, as staff must balance responding to inquiries with conducting thorough reviews.
If you have an urgent need about a submission, please contact the IRB Administrator as soon as possible.