Reporting to the IRB
What kind of events must be reported to the IRB?
The principal investigator is responsible to report to the IRB:
- Unanticipated problems
- Adverse events
- Non-compliance
- Subject complaints (e.g., issue with payments, concerns with research participation)
- Protocol deviations (e.g., any changes in approved study’s scope/process/number of subjects)
- Loss or potential loss of personally identifiable information (PII) in printed or electronic form
- Premature suspension or termination of protocol by the sponsor, investigator, or institution
Any of the above must be reported to the Oak Ridge Sitewide Institutional Review Board (ORSIRB) administrator immediately. After contacting the ORSIRB administrator, enter the information as a “Reportable New Information” for the study in IRB.
If you are not sure about an event, report it to the IRB Administrator.
For detailed information about reporting events to the IRB, refer to section “What are my obligations as a PI after IRB approval?” of the HRP-103-GENERAL-ORSIRB Principal Investigator Manual in the IRB library within the electronic IRB sytem. Also visit the DOE HSPP website regarding Reporting Requirements.
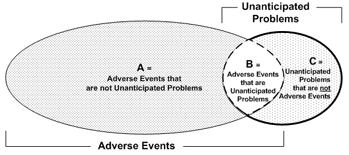
Graphic above is from OHRP Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events: OHRP Guidance (2007)
The diagram illustrates three key points:
- The vast majority of adverse events occurring in human subjects are not unanticipated problems (Area A).
- A small proportion of adverse events are unanticipated problems (Area B).
- Unanticipated problems include other incidents, experiences, and outcomes that are not adverse events (Area C).
How to Submit a Reportable Event
Once you are in the electronic IRB system, click on the Active tab. Find your study and click on the name of the study to open the study workspace. Once on the study workspace, click on “Report New Information” button. Follow the SmartForm and attach all requested documents. To submit the study, click the “Submit RNI” button on the study workspace. Maintain electronic copies of all information submitted to the IRB in case revisions are required. A job aid for reporting new information is located in the IRB library under the PI Guides tab.