Steps for Initial Submission
IRB Basic Steps

The steps below are for a new study submission. Job aids are available for creating a new study, modification, and continuing review or closure, and for reportable new information. The job aids provide more in-depth step details for submissions. The job aids can be found in the IRB library in the electronic IRB system or in the job aids.
Note: Only a principal investigator (PI) or PI proxy (staff member that can submit on behalf of the PI) can submit to the IRB. The PI proxy has to be listed as staff in the IRB submission. Alert the IRB coordinator if you want to make a staff member a PI proxy for the study.
Step 1: Is it Human Subjects Research?
Refer to the Do I need to submit to the IRB? page. Contact the IRB Administrator.
Step 2: Requirements
Be familiar with the HRP-103-GENERAL-ORSIRB Principal Investigator Manual found in the IRB library within the electronic IRB system. The HRP-103-GENERAL-ORSIRB Principal Investigator Manual provides the information needed for the researcher to successfully apply the requirements, policies and procedures of the Institution’s Human Subjects Protection Program.
Before submitting your study in the electronic IRB system, prepare your documents. The required documents can be found in the IRB library. A list of required templates can be found on the Forms and Templates page.
Training will be required for the principal investigator and any study staff. Refer to the Education and Resources page.
Step 3: Using the electronic IRB system
Log in to the electronic IRB system.
Click on the “Create New Study” button. Enter the information in the Smartform and attach all required and relevant documents for your study. Relevant documents can be consent forms, recruitment forms, surveys, interview questions, etc. Documents (e.g. protocol, consent form, recruitment, etc.) should NOT be submitted as PDFs.
All Researchers and staff must be current on their training before their study can be approved by the IRB.
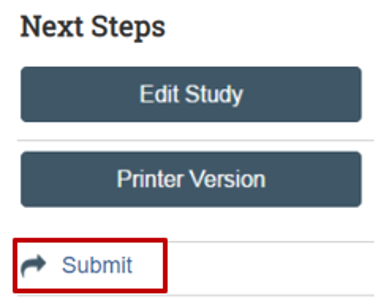
When complete, submit your study to the IRB. Saving your submission does not mean it has been submitted.
On the study workspace, click on the “Submit” button.
Step 4: After submitting to IRB
Your study has been submitted to the IRB for review. Refer to the IRB Timelines for steps the IRB takes to review your study.
Clarification Request
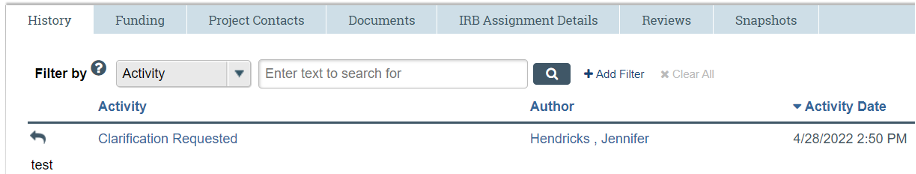
The IRB may request clarifications or revisions during the pre-review or review of a submission. Such requests will come in the form of a "Clarification Request" via email and also found under the "History" tab of the submission in the IRB system.
There are two ways to update your documents in the IRB system if you must make changes. Do NOT upload a new version so you have duplicates listed in the same section. The two ways to update documents in the system are:
- Web browser. If you need to make changes to a document already in the electronic system, do NOT delete previous version of the document. Click on the document title. See screen shot below:
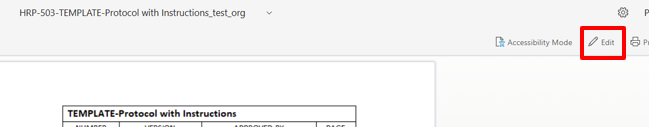
The document will open in a web browser. Click the “Edit” button located in the upper right-hand corner. Note: Headers and footers may not be correctly displayed in this view but will be normal when the document is downloaded. See screen shot below:
No changes should be tracked when using this feature and no comments should be added to the document.
- Update button. If you need to make changes to a document already in the electronic system, do NOT delete previous versions of the document. Click the “Update” button next to the document name. See screen shot below:
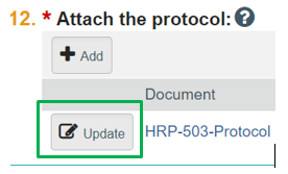
Do not upload a track-changed document. Upload the clean revised version (no markups, no comments) using the “Update button” next to the document.
Using either version to update a document will save the changes. All versions are still captured in the system history for the document.
Saving your changes does not mean it has been submitted. You must click “Submit Response” button to reply to the IRB.
The Clarification Request function is the official communication from the IRB. The study team must respond to requests for clarification within the electronic IRB within 14 days. If a response is not received in 14 days, the submission will be discarded permanently. The study submission can be recreated when the staff are ready to respond to the clarification.
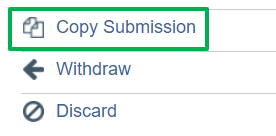
If the study is a new submission of the discarded submission, it can be copied from the original study workspace using the copy button. All content and attachments will be included in the copied submission. See screen shot below:
The IRB will not make a determination until all study team members have completed the required training.
Step 5: After Determination

The IRB will provide you with a written decision indicating the determination of IRB review.
While a study is active, make sure that training stays up-to-date for all staff.
If there are any changes to the study, a modification has to be submitted to the IRB.
A continuing review or annual review will be needed until your study is completed.
When a study is completed, a closure must be submitted to the IRB.
Any reportable event must be reported to the IRB immediately
Refer to the How to Submit to the IRB page for more information.