Proportional Counters
General
While proportional counters are most commonly used for quantifying alpha and beta activity, they are also used for neutron detection, and to some extent for X-ray spectroscopy.
The pulses produced by a proportional counter are larger than those produced by an ion chamber. This means that the proportional counter is more conveniently operated in the pulse mode (ion chambers usually operate in the current mode).
Unlike the situation in a GM detector, the pulse size reflects the energy deposited by the incident radiation in the detector gas. As such, it is possible to distinguish the larger pulses produced by alpha particles from the smaller pulses produced by betas or gamma rays.
General Types of Proportional Counters
- Gas flow proportional with window (e.g., laboratory alpha-beta counters)
- Windowless (e.g., tritium measurements)
- Air proportional (alpha counting only)
- Sealed proportional (e.g., BF3, He-3 neutron detectors)
The Size of the Pulse
The size of the pulse in a proportional counter depends on two things:
- Operating Voltage. The higher the operating voltage, the larger each avalanche becomes and the larger the pulse.
- Energy Deposited in Detector Gas. The greater the energy deposited in the detector gas by an incident particle of radiation, the larger the number of primary ion pairs, the larger the number of avalanches, and the larger the pulse.
The following diagram shows a charged particle traversing the detector gas. Four primary ion pairs (and four resulting avalanches) are produced. It is usually the case that many more ion pairs are produced by incident radiation than the four shown here. Keep in mind that the four avalanches contribute to a single pulse.
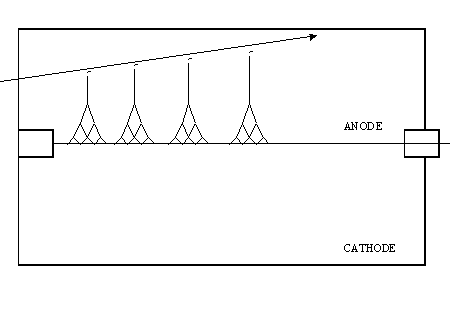
Townsend Avalanche
In a proportional counter, many electrons (10-10,000) reach the anode for each primary ion pair produced in the gas. The reason is that the electron of each primary ion pair creates further "secondary" ion pairs as it gets close to the anode. These secondary ion pairs are produced in what is called an avalanche.
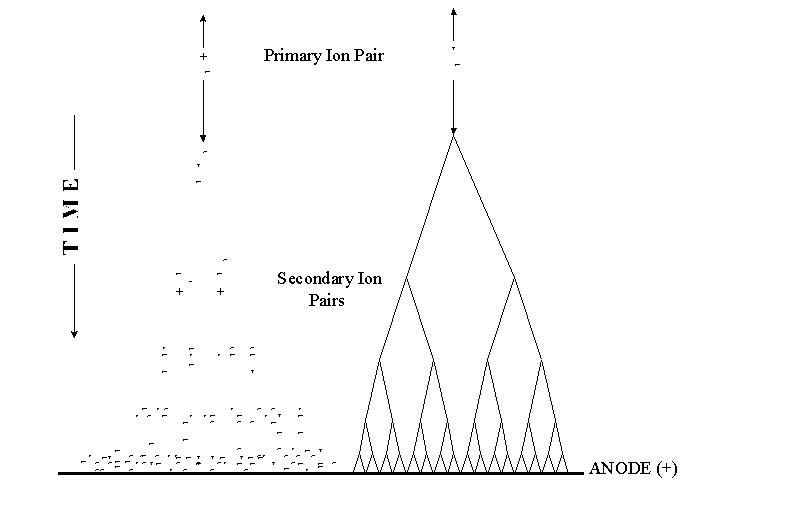
Fill Gas
In general, the proportional gas should not contain electronegative components such as oxygen. Otherwise, electrons heading towards the anode will combine with the electronegative gas. If this happens, a negative ion goes to the anode rather than an electron, and unlike the electron, the negative ion will fail to produce an avalanche. The result is that the pulse is probably too small to exceed the threshold setting and be counted.
Despite the above, air is sometimes used as a proportional gas for alpha counting. Air could not serve as a proportional gas for beta detection because beta particles produce far fewer ion pairs in the gas than alphas. Since more electrons travel towards the anode following an alpha interaction in the gas, there is a greater chance that some of them will avoid interacting with oxygen and produce an avalanche. Also, if the detector is designed so that the electrons don’t have far to travel to the anode, there is less chance that they will interact with the oxygen. Using air as the proportional gas allows the use of a thin window without the need for a gas flow system. However, it is essential that the air be dry. In high humidity conditions, air proportional counters are prone to generating spurious pulses.
The fill gas in a proportional counter (and a GM detector) is usually a noble gas because noble gases are not electronegative and don't react chemically with the detector components. Of the noble gases, argon is the most widely used because of its low cost. Other noble gases with higher atomic numbers (e.g., krypton and xenon) might be used if increased sensitivity to X-rays or gamma rays is required.
Hydrocarbon gases (e.g., methane, propane and ethylene) can also serve as a fill gas, but they have the disadvantage of being flammable.
For certain applications in dosimetry, it is desirable that a detector have the same type of response as human tissue to radiation. To accomplish this a tissue equivalent gas mixture such as the following might be used: 64.4% methane, 32.4% carbon dioxide and 3.2% nitrogen.
He-3 and BF3 are the most commonly employed gases in neutron detectors. These gases serve a dual purpose. First, thermal neutrons undergo nuclear reactions with the He-3 or BF3 to produce charged particles. Second, the charged particles ionize the He-3 or BF3 to produce pulses. The interactions between the thermal neutrons and the gas are:
n + He-3 ÿ H-3 + p+
n + B-10 ÿ Li-3 + α+
Pure noble gases can be used for alpha counting at low voltages where the multiplication factor is below 100. As a rule, however, a quench gas is added to prevent the proportional counter from acting like a geiger muller detector. During the formation of an avalanche, some gas molecules/atoms are excited rather than ionized. In other words, the energy absorbed by these proportional gas atoms/molecules promotes electrons to higher energy levels rather than frees them completely from the atoms/molecules. When the electrons deexcite and return to their original energy levels, they emit photons of visible light or UV. The problem with this is that these photons can interact with the proportional gas and cause the avalanche to spread along the anode. This can result in a non-linear relationship between the energy deposited in the detector gas and the size of the resulting pulse. These photons, particularly if they interact with the cathode wall, can also lead to the production of spurious pulses. The solution is to add a small amount of a polyatomic quench gas such as methane. The quench gas preferentially absorbs the photons, but unlike the fill gas (e.g., argon), it does so without becoming ionized.
P-10 gas, developed by John Simpson in the 1940s, is the most widely employed gas for gas flow proportional counters. It is a mix of 90% argon and 10% methane.
-
Bernstein-Ballentine Counters Bernstein-Ballentine Counters

-
Chalk River 2 Pi Proportional Counter Chalk River 2 Pi Proportional Counter

-
Chalk River 4 Pi Proportional Counter Chalk River 4 Pi Proportional Counter

-
Chalk River Anticoincidence Shields Chalk River Anticoincidence Shields

-
Early 4 pi Gas Flow Proportional Counter Early 4 pi Gas Flow Proportional Counter
-
Early Commercial 4 pi Gas Flow Proportional Counter Early Commercial 4 pi Gas Flow Proportional Counter

-
First Generation Gas Flow Alpha Proportional Counter First Generation Gas Flow Alpha Proportional Counter

-
Low Geometry Alpha Counter Low Geometry Alpha Counter

-
RCL Gas Flow Counter RCL Gas Flow Counter

-
Rossi Tissue Equivalent Proportional Counter Rossi Tissue Equivalent Proportional Counter

Neutron Detectors
Boron Trifluoride (BF3) Neutron Detectors
Paul Frame, Oak Ridge Associated Universities
General
A typical BF3 detector consists of a cylindrical aluminum (brass or copper) tube filled with a BF3 fill gas at a pressure of 0.5 to 1.0 atmospheres. The boron trifluoride gas accomplishes two things:
C it functions as the proportional fill gas.
C it undergoes an n alpha interaction with thermal neutrons: B-10 + n ÿ Li-7 + α
To improve the detection efficiency, the BF3 is enriched in B-10. Typical enrichments increase the B-10 component to 96% (ordinary boron is 20% B-10 and 80% B-11). Aluminum is typically used as the detector (cathode) wall because of its small cross section for neutrons. The anode is almost always a single thin wire running down the axis of the tube.
Pulse formation by neutrons
When a neutron is absorbed by the B-10 component of the gas, an alpha particle and a recoil Li-7 nucleus are produced that travel off in opposite directions. The movement of the alpha particle and Li-7 nucleus create primary ion pairs in the gas.
The size of the resulting pulse depends on whether the lithium nucleus was left in the ground state or an excited state. When the lithium nucleus is left in the ground state (about 6% of the time), the pulse is larger than if the nucleus were left in an excited state (about 94% of the time) because the alpha particle and Li-7 nucleus have more kinetic energy (2.792 MeV vs 2.310 MeV) with which to create ion pairs.
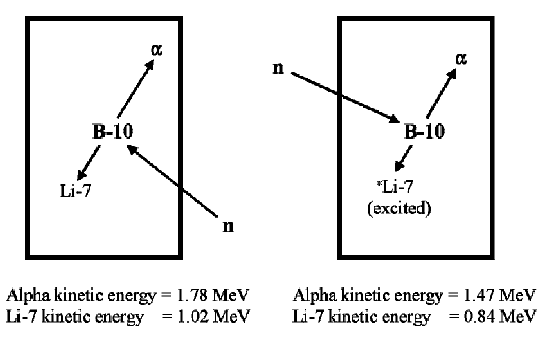
The BF3 spectrum and the wall effect
In a large diameter detector, all the kinetic energy of the alpha particle and recoil Li-7 nucleus is deposited in the detector gas. The pulse height spectrum therefore shows two peaks: a large one at 2.31 MeV (the lithium nuclei were left in an excited state) and a small one at 2.792 MeV (the lithium nuclei were left in the ground state).
For typical sized tubes (e.g., 2-5 cm diameter), smaller pulses are often produced because either the alpha particle or Li-7 nucleus deposit some of its energy in the detector wall rather than the gas. Only rarely would the alpha particle and Li-7 nucleus both strike the detector wall. If the neutron interaction takes place in the gas close enough to one side of the tube for either the alpha particle or lithium nucleus to strike the wall, the distance to the other side of the tube would be greater than the range of the particle heading towards it.
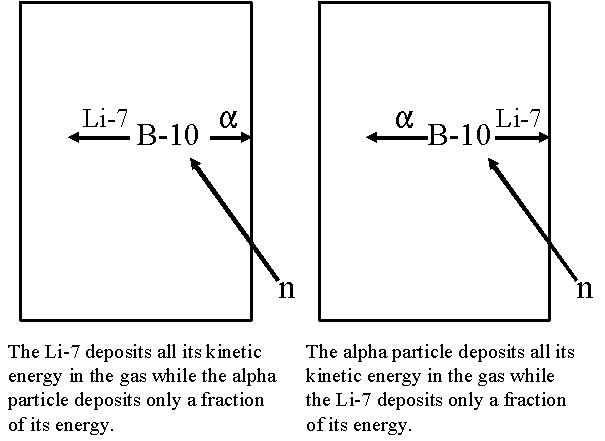
The resulting "wall effect" creates two steps on the left side of the 2.31 MeV peak. The lower step on the left is produced as a result of the alpha particle striking the wall and the Li-7 depositing all its energy (e.g., 0.84 MeV) in the gas. The higher step to the right results from the Li-7 nucleus striking the wall and the alpha particle depositing all its energy (e.g., 1.47 MeV) in the gas.
The following is a "textbook" version of the pulse height spectrum of a BF3 detector. A "real" spectrum is not quite as well defined. In particular, the two steps to the left of the 2.31 MeV peak are more difficult to distinguish than shown here.
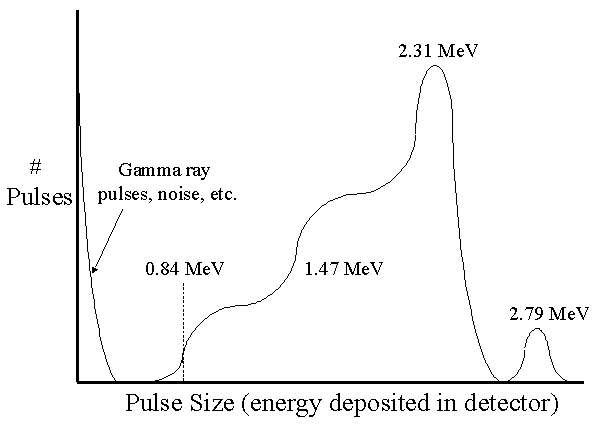
It is important to remember that this spectrum is unrelated to neutron energy—it is simply a function of the detector construction. In almost all cases, it is the count rate from the detector that carries useful information, not the pulse height.
Note that gamma rays produce relatively small pulses and these can be easily rejected by properly setting the threshold. Increasing the size of the detector reduces the wall effect seen in the above spectrum and this makes it easier to distinguish gamma pulses from those produced by neutrons.
Operating voltage and the characteristic curve
The best way to determine the operating voltage of a BF3 detector is to generate a characteristic curve (count rate versus high voltage). The curve is similar to that of a Geiger-Mueller detector in that they both have long flat plateaus. As was the case with the GM detector, the operating voltage of the BF3 detector should be selected on the plateau just above the knee. If the operating voltage is set too high, electronic noise and pulses due to background gamma rays can exceed the threshold setting and generate spurious counts. A typical voltage for a BF3 detector might be 1500 to 2000 volts.
Detection of thermal and fast neutrons
"Bare" BF3 detectors almost exclusively respond to slow (low energy) neutrons—the probability that a fast (high energy) neutron would be absorbed by boron-10 is very small. To be able to detect fast neutrons, the BF3 tube can be surrounded by a suitable moderator. The thickness of the moderator (e.g., polyethylene) might range from 1 to 6 inches depending on the neutron energy spectrum and other constraints.
-
20th Century Electronics Proportional Counter 20th Century Electronics Proportional Counter

-
20th Century Electronics Proportional Counter 20th Century Electronics Proportional Counter

-
Chalk River BF3 Proportional Counter Chalk River BF3 Proportional Counter

-
Large Chalk River BF3 Proportional Counter Large Chalk River BF3 Proportional Counter

-
Nancy Wood BF3 Proportional Counter Nancy Wood BF3 Proportional Counter

-
Nuclear-Chicago Model NC-205 BF3 Counter Nuclear-Chicago Model NC-205 BF3 Counter

-
RCL Mark 2, Model 1 Proportional Counter RCL Mark 2, Model 1 Proportional Counter

-
RCL Mark 2, Model 201 Proportional Counter RCL Mark 2, Model 201 Proportional Counter

