Quadrant Electrometers
Quadrant Electrometers
Paul Frame, Oak Ridge Associated Universities

The primary function of an electrometer is to measure an electrical potential (volts) or charge (coulombs)—charge and electric potential are directly related. By measuring the change in the accumulated charge over time, the current (amperes) can also be determined. One ampere is equivalent to one coulomb per second.
The basic quadrant electrometer was developed by Lord Kelvin (William Thomson) in the 1860s. The earliest versions were usually housed inside a “bird cage” or box. The bird cage electrometers got their name from the Faraday cage that was used to protect the instrument from stray electrostatic charges. In some cases, a glass bell jar protected them from the air currents which could affect their operation. The other common approach was to house the electometer inside a wooden box, the front of which was made of glass.
However, it wasn’t until the development of the Dolezalek quadrant electrometer in 1896 that a device was available with the sensitivity needed to measure the very small currents (picoamps) associated with the ionization chambers used to measure radioactive samples. Among other things, the Dolezalek electrometer and its later derivations protected the quadrants inside a cylindrical metal housing that had one or possibly two small glass windows.

Quadrant electrometers could be operated in a number of ways but the most common method was to measure the rate of deflection of the electrometer’s vane (aka needle) suspended by a fine wire or fiber.
The potential being measured caused a butterfly-shaped vane (ca. 1 – 3” across) to turn. The magnitude of this deflection was related to the potential (or charge). The rate of the deflection was related to the current. The early electrometers used vanes that were solid metal and relatively heavy. In contrast, the vane of the Dolezalek electrometer was very thin and light—it was sometimes made of aluminum, sometimes of metal-coated paper. One end of a short rigid wire was connected to the middle of the vane while the other end of the wire had a small hook. The backside of a tiny mirror (a few mm in diameter) was attached roughly half way up the wire. The hook was connected to the end of a thin flexible wire or fiber so that the vane and mirror were suspended in air and free to rotate.
The vane itself was inside, but not in physical contact with, a metal “pill box” shaped device consisting of four quadrants. In the photo on the left, the quadrants have been opened up so that the vane is visible.
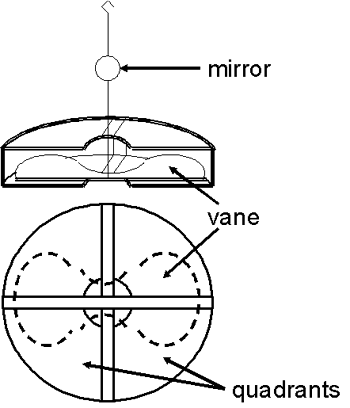
Each quadrant was electrically connected to the quadrant diagonally opposite it so that they had the same charge. One pair of quadrants had a positive charge and the other pair had a negative charge. The electrical charge on the vane caused it to take up a particular orientation within the quadrants. If the potential difference between the vane and the quadrants changed (e.g., due to current from the ion chamber), the vane and the mirror would rotate.
Prior to the measurement, the system was adjusted (mechanically and electrically) so that the vane was positioned inside the quadrants as shown in the accompanying figure. Half of each lobe of the vane was inside one quadrant while the other half was inside the adjacent quadrant.
To determine the position of the vane, a beam of light was shone through the window in the electrometer case so that it reflected off the mirror onto a scale usually positioned one meter away. As the current from the ion chamber changed the potential difference between the vane and the quadrants, the vane rotated and the reflected beam of light moved across the scale. The time required to move across a specified number of divisions on the scale could be related to the activity of the sample by calibration with a known source. In some cases, a calibration wasn’t necessary, only the relative rates of deflection under different experimental conditions might have been of interest.
Early Quadrant Electrometers
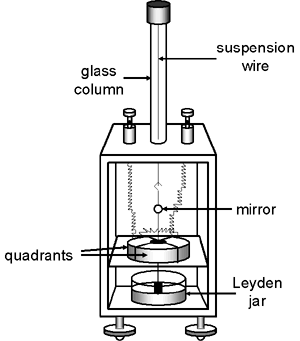
Note that the quadrant electrometers of the 1800s did not provide the sensitivity or the reliability that was required for radioactive work.
The early bird cage and box-like quadrant electrometers maintained the vane at a constant potential while the potential/charge being measured was applied to one pair of quadrants. The other pair of quadrants was grounded. The mechanism employed to maintain the potential on the vane was somewhat cumbersome: a wire was suspended beneath the vane so that the free end at the bottom (usually attached to some sort of weight) was immersed in a sulfuric acid solution. The sulfuric acid was contained inside a Leyden jar to which a charge had been applied. A Leyden jar, actually an early form of capacitor, is simply a glass jar, the bottom portion of which is lined on the inside and outside with metal foil. The sulfuric acid provided the electrical connection between the foil on the inside of the jar and the wire connected to the electrometer vane.
Dolezalek Electrometer
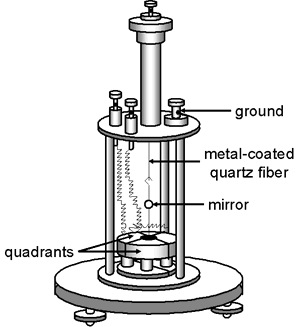
The Dolezalek electrometer, invented by the Hungarian, Friedrich Dolezalek (1873-1920), represented a significant improvement over earlier versions of quadrant electrometers by virtue of its increased sensitivity. It was invented in the same year that radioactivity was discovered (1896) and it quickly became a favorite of those investigating radioactive substances (e.g., Ernest Rutherford). Dolezalek spent most of his career in Germany and his research spanned a variety of fields: physics, chemistry, and electrical engineering.
The Dolezalek quadrant electrometer differs from previous designs in several respects:
- The vane is lighter. It was usually made of paper coated with a thin layer of metal (e.g., silver) although thin aluminum was sometimes used. Older vanes were solid metal.
- The quadrants are smaller.
- The mirror and vane were suspended by a metal coated quartz fiber rather than a phosphor-bronze strip.
- The Leyden jar beneath the quadrants has been eliminated.
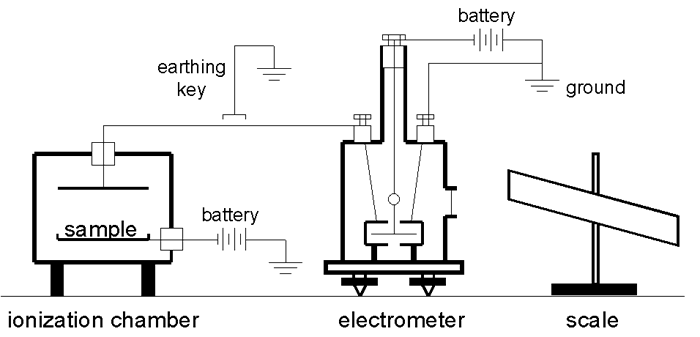
The following diagram shows how a Dolezalek electrometer and an ionization chamber might have been configured to measure the activity of a radioactive substance. In this example a fixed potential (e.g., 100-200 volts) is maintained between one pair of quadrants (grounded) and the vane. The charge collected by the ionization chamber accumulated on the other pair of quadrants.
Major Types of 20th Century Quadrant Electrometers
- Dolezalek
- Compton - a variant of the Dolezalek electrometer. The major difference being that the Compton electrometer permitted a mechanical adjustment of the position of one of the quadrants.
- Hoffman - the most sensitive of the quadrant electrometers. Actually, it was a binant (rather than quadrant) electrometer that used a vane with a single lobe. It employed heat sinks to reduce thermally generated air currents that could affect the vane and it was partially evacuated so that it operated at reduced pressure (a few millimeters of mercury).
-
Cambridge Dolezalek Electrometer Cambridge Dolezalek Electrometer

-
Dolezalek Electrometer Dolezalek Electrometer

-
Dolezalek Electrometer Dolezalek Electrometer

-
Galvanometer Scale Galvanometer Scale

-
Griffin & Tatlock Quadrant Electrometer Griffin & Tatlock Quadrant Electrometer

-
Harris Quadrant Electrometer Harris Quadrant Electrometer

-
Large Dolezalek Electrometer Large Dolezalek Electrometer

-
W.G. Pye & Co. Dolezalek Electrometer W.G. Pye & Co. Dolezalek Electrometer

