Human Research Protection Program
In 1967, Oak Ridge Associated Universities (ORAU) formally established a Committee on Human Studies to review ORAU’s biomedical research proposals involving human subjects. In 1999, a single independent IRB in Oak Ridge, named Oak Ridge Sitewide Institutional Review Board (ORSIRB), was created. ORAU established and maintains the ORSIRB in accordance with the DOE Order 443.1C, and 10 CFR 745.

ORSIRB serves as the IRB of record for study protocols for Oak Ridge Associated Universities (ORAU), Oak Ridge National Laboratory (ORNL), Y-12 National Security Complex (Y-12) other DOE facilities under of the purview of the Oak Ridge Site Office. Each site has their own Institutional Officials and each site contributes to the ORSIRB. The ORSIRB is part ORAU’s Human Research Protection Program (HRPP) which is accredited by the Association of Accreditation of Human Research Protection Programs (AAHRPP), an independent, non-profit accrediting body, that ensures HRPPs meet rigorous standards for quality and protection.
HRPP components
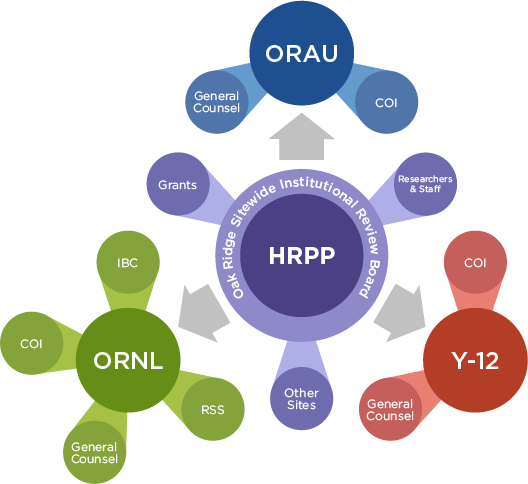
The HRPP is comprised of many elements that work together to operate a protection program for human subjects. The HRPP is not the IRB alone but all the elements within the organization that work together to provide the highest possible standard of protection for each subject in a research study.
ORSIRB
- Oak Ridge Associated Universities (ORAU) is a 501(c)(3), not-for-profit organization that has been working with government agencies, universities, and corporate entities since 1946. ORAU has partnered with the U.S. Department of Energy (DOE) and other government agencies to advance national priorities in science, education, security and health. In 1967, ORAU formally established a committee to review human subject studies and in 1999, a single independent IRB named ORSIRB was created.
- Oak Ridge National Laboratory (ORNL) is the largest Department of Energy science and energy National Laboratory in the Nation, advances discoveries and their translation into energy and national security solutions. Since its origins over 80 years ago, ORNL has empowered leaders and teams to advance breakthroughs that have had international scale impact in an environment marked by operational excellence and engagement with the communities where we live and work. ORNL has been conducting human research projects since 1970.
- Institutional Biosafety Committee (IBC)
- Office of Research Integrity (ORI)
- The Y-12 National Security Complex has three primary national security missions that protect our country and our allies around the world. Maintaining the U.S. nuclear stockpile, reducing global threats, and fueling the U.S. Nuclear Navy are key activities.
Conflict of Interest (COI)
Conflicts of interest (COI) validation is completed for each study. If a COI is identified for any team research member, the General Counsel’s Office of that organization’s research staff is consulted to develop a COI management plan to be used for the study.
General Counsels
The General Counsel’s Office at each organization has an active role in many elements of protecting human research participants. These offices are engaged when needed to assist with review of Data Use Agreements, manage conflicts of interest, review updated regulatory guidance, and more.
Researchers and staff
Researchers and staff from each organization from within the HRPP make up an important part of our program. These individuals are committed to compliance and ethical treatment of participants and/or the human data and specimens used in the research each organization conducts. Research and staff engagement is paramount in the operation of a successful HRPP.
Grants
Each institution manages any received grants with appropriate elements (General Counsel’s Office, Strategic Partnerships Program, etc.) within the organization’s HRPP with support from the IRB office when needed.
Other sites that fall under the Oak Ridge Site Office
If needed, the Oak Ridge Site Office could (in the future) assign additional organizations or functions to expand the scope of the HRPP.
HRPP key information
ORAU has an approved Federalwide Assurance (FWA) with the U.S. Department of Health & Human Services (HHS) and is also registered with the HHS Office for Human Research Protections (OHRP).
- ORAU FWA number: FWA00005031
- ORNL FWA number: FWA00012860
- Y-12 FWA number: FWA00031760
- IRB registration number: IRB00000547