Human Research Protection Program
In 1967, Oak Ridge Associated Universities (ORAU) formally established a Committee on Human Studies to review ORAU’s biomedical research proposals involving human subjects. In 1999, a single, independent institutional review board (IRB) in Oak Ridge, named the Oak Ridge Sitewide Institutional Review Board (ORSIRB), was created. ORSIRB serves as the IRB of record for study protocols for ORAU, Oak Ridge National Laboratory (ORNL), Y-12 National Security Complex (Y-12), and other DOE facilities under of the purview of the Oak Ridge Site Office. Each site has its own institutional officials and contributes to the ORSIRB.
ORAU is responsible for operation of the Human Research Protection Program (HRPP). This program is comprised of the many elements that work together to operate a protection program for human subjects. Our HRPP is accredited by the Association of Accreditation of Human Research Protection Programs, an independent, non-profit accrediting body, that ensures that HRPPs meet rigorous standards for quality and protection.
The HRPP is not the IRB alone but all the elements within the organization that work together to provide the highest possible standard of protection for each subject in a research study.
HRPP components
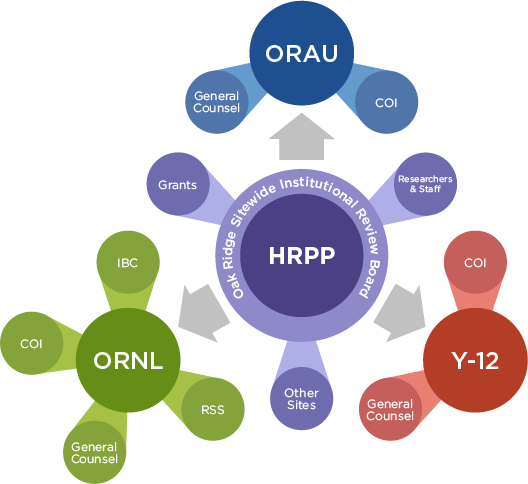
- ORSIRB
- ORAU
- ORNL
- Institutional Biosafety Committee (IBC)
- Office of Research Integrity (ORI)
- Y-12
- Conflict of Interest (COI)
- General Counsels
- Other sites that fall under the Oak Ridge Site Office
- Researchers and staff
- Grants
HRPP key information
ORAU has an approved Federalwide Assurance (FWA) with the U.S. Department of Health & Human Services (HHS) and is also registered with the HHS Office for Human Research Protections (OHRP).
- ORAU FWA number: FWA00005031
- ORNL FWA number: FWA00012860
- Y-12 FWA number: FWA00031760
- IRB registration number: IRB00000547